What is the difference between cis and trans?
Sommario
- What is the difference between cis and trans?
- Is cis or trans favored?
- What is cis and trans configuration?
- What is the difference between CIS trans and EZ?
- What is trans configuration?
- What is the difference between cis trans and EZ?
- What are cis and trans alkenes?
- Is E-Z the same as CIS trans?
- Is E isomer cis or trans?
- What are cis and trans fatty acids?
- What does CIS and trans mean in chemistry?
- Why is trans more stable than cis?
- What is cis and trans configuration?
- What is the difference between cis and trans isomers?
What is the difference between cis and trans?
Cisgender, or cis, means that the gender you identify with matches the sex assigned to you at birth. ... In Latin, “cis” means “on this side,” while “trans” means “on the other side.” A transgender woman had male genitals at birth but identifies as female.
Is cis or trans favored?
In most cases, the trans isomer is favored over the cis isomer. The reason for this is that the cis isomer places the R groups of the two connected amino acids in close proximity to each other.
What is cis and trans configuration?
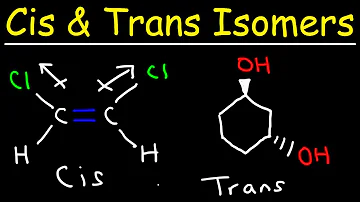
Cis–trans isomerism, also known as geometric isomerism or configurational isomerism, is a term used in organic chemistry. ... In the context of chemistry, cis indicates that the functional groups (substituents) are on the same side of some plane, while trans conveys that they are on opposing (transverse) sides.
What is the difference between CIS trans and EZ?
They are the same, E- ≡ trans- and Z- ≡ cis- . Except E-Z is used for more complex isomers. As each side only has 1 H and 1 Cl atom, you can tell if the Cl atoms are on the same or opposite sides, and therefore decide upon cis- or trans-. ... As the heaviest 'corner' on each side is on opposite sides, it is an E- isomer.
What is trans configuration?
trans configuration: configuration of a geometrical isomer in which two groups are on opposite sides of an imaginary reference line on the molecule.
What is the difference between cis trans and EZ?
They are the same, E- ≡ trans- and Z- ≡ cis- . Except E-Z is used for more complex isomers. As each side only has 1 H and 1 Cl atom, you can tell if the Cl atoms are on the same or opposite sides, and therefore decide upon cis- or trans-. ... As the heaviest 'corner' on each side is on opposite sides, it is an E- isomer.
What are cis and trans alkenes?
Consider the longest chain containing the double bond: If two groups (attached to the carbons of the double bond) are on the same side of the double bond, the isomer is a cis alkene. If the two groups lie on opposite sides of the double bond, the isomer is a trans alkene.
Is E-Z the same as CIS trans?
We often use cis/trans for convenience, but E/Z is the “official”, IUPAC approved way to name alkene stereoisomers]. One easy way to remember Z is to say “Zee Zame Zide” in a German accent.
Is E isomer cis or trans?
E (from the German entgegen) means "opposed" in the sense of "opposite". That is, Z has the higher-priority groups cis to each other and E has the higher-priority groups trans to each other.
What are cis and trans fatty acids?
Cis fatty acids are formed using double bonds that are incorporated with hydrogen atoms. On the other hand, trans fatty acids are formed with double bonds that are incorporated with carbon atoms. Cis fatty acids are made in the natural process.
What does CIS and trans mean in chemistry?
- In geometrical isomer nomenclature, the prefix cis- and trans- are used to identify which side of the double bond the similar atoms are found. The cis- prefix is from the Latin meaning "on this side". In this case, the chlorine atoms are on the same side of the carbon-carbon double bond.
Why is trans more stable than cis?
- Trans isomers are usually more stable than cis isomers because there is no steric interaction, or repulsion, between the carboxyl groups of the molecule. The more negative heat of formation value of trans alkenes also show that these compounds are more stable than cis alkenes.
What is cis and trans configuration?
- The cis and trans configurations are considered examples of geometric isomerism or configurational isomerism. Cis and trans should not be confused with E/Z isomerism. E/Z is an absolute stereochemical description only used when referencing alkenes with double bonds that cannot rotate or ring structures.
What is the difference between cis and trans isomers?
- Cis-trans isomerism can be found when the position of a side group is changed while the rest of the molecules are identical to each other. The main difference between cis and trans isomers is that cis isomers are essentially polar whereas trans isomers are comparatively nonpolar.














