What type of bond is van der Waals?
Sommario
- What type of bond is van der Waals?
- What are the 3 types of van der Waals forces?
- What is van der Waals famous for?
- What is an example of a van der Waals interaction?
- What is Kessome attraction?
- What molecules are the Van der Waals in?
- What is the difference between van der Waals and London dispersion?
- What are van der Waals constants?
- Who invented van der Waals?
- What is van der Waals forces for kids?
- Are van der Waals and London dispersion the same thing?
- What is van der Waals force of attraction?
- Why are hydrogen bonds stronger than van der Waals forces?
- Why do van der Waals forces increase down a group?
What type of bond is van der Waals?
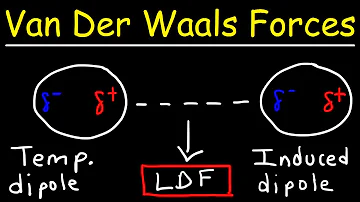
Van der Waals bond: A weak attractive force between atoms or nonpolar molecules caused by a temporary change in dipole moment arising from a brief shift of orbital electrons to one side of one atom or molecule, creating a similar shift in adjacent atoms or molecules.
What are the 3 types of van der Waals forces?
van der Waals forces may be classified into three types: electrostatic, induction, and dispersion. Most textbooks only mention the most important interaction in each class, that is, the dipole–dipole, dipole-induced dipole, and London dispersion contributions, as these are always significant when they occur.
What is van der Waals famous for?
listen); 23 November 1837 – ) was a Dutch theoretical physicist and thermodynamicist famous for his pioneering work on the equation of state for gases and liquids. Van der Waals started his career as a school teacher.
What is an example of a van der Waals interaction?
Examples of van der Waals forces include hydrogen bonding, dispersion forces, and dipole-dipole interactions.
What is Kessome attraction?
Keesom interactions are the electrostatic interactions among the permanent dipoles of polar molecules. They arise when the δ+ end of a dipole in one molecule is attracted to the δ- end of a dipole in another molecule.
What molecules are the Van der Waals in?
A Van der Waals molecule is a weakly bound complex of atoms or molecules held together by intermolecular attractions such as Van der Waals forces or by hydrogen bonds. The name originated in the beginning of the 1970s when stable molecular clusters were regularly observed in molecular beam microwave spectroscopy.
What is the difference between van der Waals and London dispersion?
Glossary. London dispersion forces: The intermolecular forces that occur between atoms and between nonpolar molecules as a result of the motion of electrons. Van der Waals forces: The weakest intermolecular force and consist of dipole-dipole forces and dispersion forces .
What are van der Waals constants?
The constants a and b are called van der Waals constants. They have positive values and are characteristic of the individual gas. If a gas behaves ideally, both a and b are zero, and van der Waals equations approaches the ideal gas law PV=nRT. The constant a provides a correction for the intermolecular forces.
Who invented van der Waals?
physicist Johannes Diderik van der Waals The forces are named for the Dutch physicist Johannes Diderik van der Waals, who in 1873 first postulated these intermolecular forces in developing a theory to account for the properties of real gases.
What is van der Waals forces for kids?
Van der Waals' forces are the weakest type of intermolecular force. They are named after the Dutch scientist Johannes Diderik van der Waals (1837–1923). Negatively charged electrons orbit molecules or ions. The electrons create slightly different charges from one end of the molecule to the other.
Are van der Waals and London dispersion the same thing?
- So the Casimir and London (dispersion) forces are the same thing at the fundamental level. ("van der Waals" is an ambiguous term that may refer to other interactions, like permanent dipole interactions).
What is van der Waals force of attraction?
- In simple words, Van Der Waals Forces are those bonds that play the role of attracting both molecules and atoms. These interactions include weak electrostatic forces lying in a close range within molecules lacking charges. Moreover, they are the weakest intermolecular forces, comprising of dipole-dipole and dispersion forces.
Why are hydrogen bonds stronger than van der Waals forces?
- Hydrogen bonding , an interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons. Such a bond is weaker than an ionic bond or covelant bond but stronger than van der Waals forces.(Ref:@Brittanica/hydrogen bonding).
Why do van der Waals forces increase down a group?
- Boiling and melting points increase as you go down the group. This is because the strength of the van der Waals forces increases since the atoms have more electrons as you descend the group.














